|
|
|
|
 In
its purest form, it's odorless, nearly colorless and tasteless. It's in
your body, the food you eat and the beverages you drink. You use it to
clean yourself, your clothes, your dishes, your car and everything else
around you. You can travel on it or jump in it to cool off on hot summer
days. Many of the products that you use every day contain it or were
manufactured using it. All forms of life need it, and if they don't get
enough of it, they die. Political disputes have centered around it. In
some places, it's treasured and incredibly difficult to get. In others,
it's incredibly easy to get and then squandered. What substance is more
necessary to our existence than any other? Water. In
its purest form, it's odorless, nearly colorless and tasteless. It's in
your body, the food you eat and the beverages you drink. You use it to
clean yourself, your clothes, your dishes, your car and everything else
around you. You can travel on it or jump in it to cool off on hot summer
days. Many of the products that you use every day contain it or were
manufactured using it. All forms of life need it, and if they don't get
enough of it, they die. Political disputes have centered around it. In
some places, it's treasured and incredibly difficult to get. In others,
it's incredibly easy to get and then squandered. What substance is more
necessary to our existence than any other? Water.
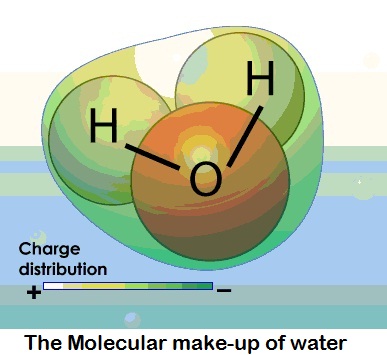
At its most basic, water is a molecule with one oxygen atom and two
hydrogen atoms, bonded together by shared electrons. It is a V-shaped
polar molecule, which means that it's charged positively near the
hydrogen atoms and negatively near the oxygen atom. Water molecules are
naturally attracted and stick to each other because of this polarity,
forming a hydrogen bond. This hydrogen bond is the reason behind many of
water's special properties, such as the fact that it's denser in its
liquid state than in its solid state (ice floats on water).
Water is the only substance that occurs naturally as a solid (ice), a
liquid and a gas (water vapor). It covers about 70 percent of the Earth
for a total of approximately 332.5 million cubic miles (1,386 million
cubic kilometers). If you're familiar with the lines "Water, water,
everywhere, nor any drop to drink" from the poem "The Rime of the
Ancient Mariner," you'll understand that most of this water -- 97
percent of it -- is undrinkable because it's saltwater. Only 3 percent
of the world's water supply is freshwater, and 77 percent of that is
frozen. Of the 23 percent that is not frozen, only a half a percent is
available to supply every plant, animal and person on Earth with all the
water they need to survive.
So water is pretty simple, right? Actually, there are a lot of things
about it that scientists still don't fully understand. And the problem
of making sure that enough clean, drinkable water is available to
everyone and everything that needs it is anything but simple. In this
article, we'll look at some of these problems. We'll also explore
exactly what plants, animals and people do with water and learn more
about what makes water so special.
Source: http://science.howstuffworks.com |
|


 In
its purest form, it's odorless, nearly colorless and tasteless. It's in
your body, the food you eat and the beverages you drink. You use it to
clean yourself, your clothes, your dishes, your car and everything else
around you. You can travel on it or jump in it to cool off on hot summer
days. Many of the products that you use every day contain it or were
manufactured using it. All forms of life need it, and if they don't get
enough of it, they die. Political disputes have centered around it. In
some places, it's treasured and incredibly difficult to get. In others,
it's incredibly easy to get and then squandered. What substance is more
necessary to our existence than any other? Water.
In
its purest form, it's odorless, nearly colorless and tasteless. It's in
your body, the food you eat and the beverages you drink. You use it to
clean yourself, your clothes, your dishes, your car and everything else
around you. You can travel on it or jump in it to cool off on hot summer
days. Many of the products that you use every day contain it or were
manufactured using it. All forms of life need it, and if they don't get
enough of it, they die. Political disputes have centered around it. In
some places, it's treasured and incredibly difficult to get. In others,
it's incredibly easy to get and then squandered. What substance is more
necessary to our existence than any other? Water.